
Twenty-six years ago, the West Nile virus (WNV) invaded New York, infecting and killing birds, horses, humans, and other animals. An estimated 25 horses were diagnosed with WNV in 1999, and as the virus spread rapidly across the U.S., this number skyrocketed, peaking in 2002 at over 15,000 confirmed equine cases. Thanks to rapid vaccine development and administration in 2003, the number of horses diagnosed with WNV quickly fell. Over the past 15 years, confirmed WNV cases reported by the Animal and Plant Health Inspection Service (APHIS) averaged about 265 per year, with as few as 71 cases in 2020 and as many as 627 cases in 2012.
Angela Pelzel-McCluskey, DVM, MS, Equine Epidemiologist, Strategy and Policy, USDA-APHIS-Veterinary Services, in Fort Collins, Colorado, assures us, however, that WNV is still relevant and more rampant than the numbers let on.
“We know there is significant underreporting of cases,” she says. “The cases being reported on the (USDA’s) arboviral dashboard go through the Centers for Disease Control and are laboratory diagnosed, meaning the horse’s owner paid for the diagnostic testing.”
Ideally, all neurologic cases would be fully worked up; however, financial constraints often limit our ability to do so.
“Owners that didn’t have the $25 to vaccinate against WNV won’t have the $300-400 for laboratory testing,” Pelzel-McCluskey adds. “We probably have a lot of practitioners that see likely neurologic horses and know the horse is either undervaccinated or not vaccinated at all, but they just aren’t able to test.”
In addition, we’re also likely missing the WNV-positive horses that are asymptomatic or only have mild symptoms and, therefore, aren’t tested.
Alan Goodman, PhD, an associate professor from the School of Molecular Biosciences at Washington State University, echoes Pelzel-McCluskey’s thoughts on the continued relevance of WNV. “WNV is the most common mosquito-borne pathogen in the United States,” he says.
Feng Li, DVM, PhD, the William Robert Mills Endowed Chair in virology at the University of Kentucky’s Gluck Equine Research Center, adds, “WNV will likely become even more widespread due to global climate change that may alter viral transmission and replication rates. Changing weather conditions may change the habitat of the mosquito species that can be infected with WNV and may also affect how well these mosquitoes can transmit the virus from one animal to another.”
Another reason WNV is still relevant is because humans are also at risk of infection, and we share the same environment.
“In areas where we have WNV in horses, we also have a higher human caseload or even spillover into other species like wild animals,” says Pelzel-McCluskey. “Mosquitoes do feed on both humans and horses, so heavy mosquito strike and lots of virus means lots of infections in humans also. And the number of human cases lately is very concerning.”
West Nile Refresher
WNV is a flavivirus, which is the same family as other vector-borne diseases. Infected mosquitoes transmit the virus to birds during blood feeding. Birds are natural reservoirs or amplifying hosts, which means virus levels in the blood can become high enough that when another mosquito feeds on that bird, the mosquito can become infected and then transmit the virus to another bird. While horses and humans can become infected with WNV after transmission from an infected mosquito, they’re considered dead-end hosts. Virus levels do not become high enough in humans and horses that another mosquito can become infected when feeding on them.
After a mosquito bites a horse, the virus can replicate in the skin cells in the vicinity of the bite and spread to other cells. In horses with severe clinical signs related to neuroinvasive disease, the virus has been able to spread to neuronal tissues such as the spinal cord and the brain. The infection of these neuronal tissues ultimately leads to the damage and death of brain cells (neurons), which causes the horse to show neurologic signs.
According to Li and Lianne Eertink, his PhD candidate studying equine virology, most WNV infections are asymptomatic, meaning the body clears the infection without any clinical signs. Only an estimated 10-39% of horses develop clinical signs of infection.
“We know this based on experimental studies done on horses where groups of horses were infected with WNV and researchers observed how many animals developed clinical signs,” they explain.
Experimental studies also show that the incubation period (from the time of infection to development of clinical signs) is about seven to nine days.
Being a neuroinvasive disorder, the clinical signs of infection, from most to least common, include:
- Ataxia.
- Muscle weakness and paralysis.
- Fever.
- Anorexia and lethargy.
- Abnormal behavior, including teeth grinding and circle walking.
- Cranial nerve deficits such as blindness, difficulty swallowing, and facial paralysis.
Who Is at Risk of Infection?
Historically, we thought horse breed was a risk factor for infection, but more recent studies suggest management is more likely to be the true risk factor, with certain breeds being managed differently. According to Ganzenberg et al. (2020), horses housed outdoors full time are 2.63 times more likely to be infected than horses housed outdoors less than 24 hours per day. Interestingly, that study also found that horses with fly sheets are 7.22 times more likely to be infected than horses not wearing fly sheets.
“The article does not provide an explanation for this finding, but in my opinion, it has likely to do with the management of horses that are provided a fly sheet compared to horses that are not provided a fly sheet,” says Li. “I would think that horses with fly sheets are generally turned out more than horses that never wear fly sheets (which is related to the findings about full-time vs. part-time outdoor housing). In addition, horses in this study that wore a fly sheet might have been in more insect-dense regions than the horses that didn’t need one in this study. In that case, more insects means more mosquitoes, which means more WNV.”
Another potential risk factor is related to the OAS1 gene that encodes a protein called 2’-5’-oligoadenylate synthetase 1.
“This is an interferon-induced effector protein that can protect horses against WNV and other viral infections by recognizing viral RNAs and sending them for degradation,” Li explains. “Due to naturally occurring mutations in the OAS1 protein in different horse populations, some genetic mutations in the OAS1 gene may affect the horse’s ability to disarm WNV infectivity and mitigate the associated encephalitis.”
Li and Eertink say that in addition to the OAS1 gene, several interferon-induced antiviral effector proteins, such as ISG15 (IFN-stimulated protein of 15 kDa), the Mx (myxovirus resistance) proteins, and protein kinase R (PKR), might play similar roles in neutralizing WNV infection in horses and reducing the severity of encephalitis.
“Again, genetic polymorphisms for these antiviral proteins in horses may impact the susceptibility of horses to WNV infection,” says Li.
Diagnosing West Nile Virus
Major differential diagnoses to consider when faced with a neurologic horse include rabies, equine protozoal myeloencephalitis (EPM), other arboviral diseases (Eastern and Western encephalitis viruses), and the neurologic form of equine herpesvirus-1 (equine herpesvirus myeloencephalopathy), among others.
“The recommended test for diagnosing WNV in live horses is the IgM capture ELISA on a blood sample,” says Pelzel-McCluskey. “This test is widely available at commercial laboratories and is a good test for neurologic horses that have not been vaccinated against WNV in the previous 30 days.”
Treatment and Recovery
There are no targeted therapies for WNV. Affected horses are managed with supportive care, including slings to support horses unable to stand, non-steroidal anti-inflammatory drugs for controlling inflammation and pain, treating injuries secondary to recumbency or thrashing, and maintaining hydration and nutrition.
Mortality rates are reportedly as high as 71% but typically in the 22-36% range, equivalent to one in every three to four affected horses. Older horses, foals under 12 months, and immunocompromised animals have higher mortality rates.
“These mortality rates are in horses that developed neuroinvasive disease,” says Li. “The mortality rate of the total number of horses infected with WNV, which includes asymptomatic horses, horses with only mild symptoms, and horses with neuroinvasive disease, is much lower.”
Further, approximately 10-20% of horses have residual neurologic deficits following recovery. But, about 90% of those residual deficits resolve within 12 months postinfection.
Preventing West Nile Virus Infection
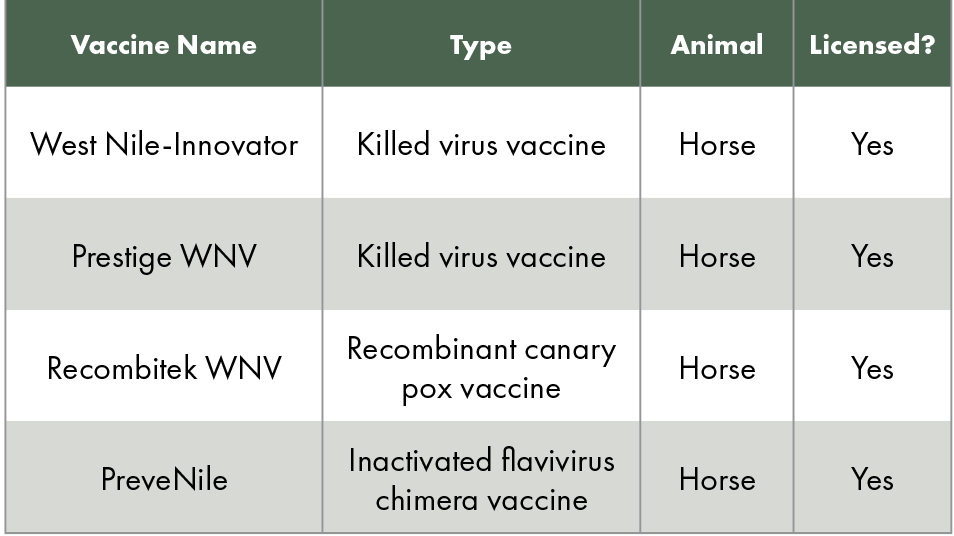
Unlike human medicine, veterinarians have several vaccines to choose from to protect horses against WNV. In fact, veterinarians can choose from four different vaccines (see Table 1). According to the American Association of Equine Practitioners (AAEP), WNV is a core vaccine, recommended for all horses with annual revaccination. The AAEP also recommends vaccinating at the start of vector season—for example, spring in the Northeastern United States—and every six months in endemic areas.
“However, climate change is altering vector season, and we are seeing mosquito-borne diseases in February in states that normally don’t have mosquitoes at that time of year, like South Carolina,” Pelzel-McCluskey says.
The AAEP also recommends implementing mosquito control strategies. This includes applying insect repellents containing permethrin daily during vector season, especially at dawn and dusk when mosquitoes are most active. Further, owners can avoid turning horses out during these times and use fans to repel mosquitoes from stalled horses.
“With the fly sheets, we need to really look at whether we are truly covering the areas where the mosquitoes are feeding,” Pelzel-McCluskey stresses. “The legs aren’t covered, and if owners are using fly sheets, they may be trying to minimize their use of permethrin repellents. To be effective, we need a complete and robust program to keep biting insects off the horse that deals with the manure pile, standing water, and protecting all parts of the horse’s body.”
The Future of Mosquito Control
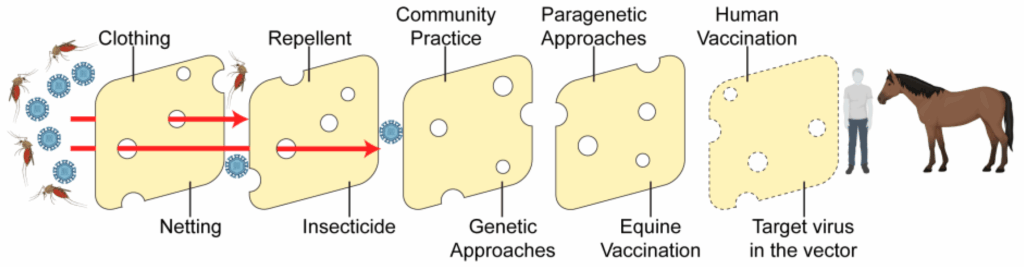
In the U.S, more than 65 mosquito species are known to be infected with WNV. The primary vectors, however, are Culex pipiens, C. tarsalis, and C. quinquefasciatus (Feng et al. 2024).
In his 2024 article highlighting control methods for WNV, Goodman recommends “integrative vector management,” which includes source, larval, and adult control, both pro- and reactive.
“An example of proactive is using insect repellent, mosquito netting, and dumping out standing water,” he explains. “An example of reactive is using insecticide after a prevalence of infected mosquitoes are detected in a region.”
- Vector management includes:
- Using small crustaceans in ponds or ditches to control mosquito larval populations.
- Governments applying pesticides to public lands.
- Using streetlamps that do not attract insects in residential areas.
“Effective mosquito control techniques for both horses and humans would be broadly deployable, utilized proactively, and avoid environmental concerns such as using pesticides and disrupting waterways,” says Goodman. “Whenever possible, the control measures would be specific to mosquitoes or the pathogens they carry without impacting other insects or beneficial microbes.”
An interesting area of research is genetic mosquito control, which meets these criteria. Researchers are exploring two genetic approaches: refractory and lethal. Refractory mosquito techniques involve introducing a gene or gene drive into a mosquito that is involved in the insect’s immune response to WNV. This target gene is then either up- or downregulated to enhance immune responses to ultimately lower the viral load and, therefore, reduce WNV transmission. This target gene would need to be passed on to progeny, so the system is self-sustaining.
Alternatively, mosquito genes can be mutated to have a desired lethal effect in the population. “An example of this is known as the sterile insect technique (SIT),” says Goodman. “Insects are exposed to ionizing radiation to induce male sterility. Sterile males are released into the wild to mate with females, and the result is nonviable progeny. Repeated releases of sterile males can suppress or eliminate desired mosquito populations.”
He adds, “While the techniques described above are still in development for WNV-carrying Culex mosquitoes, they have been used successfully in mosquitoes that transmit malaria and dengue virus. Another hurdle to the use of genetic control measures for mosquitoes is community understanding. Broad messaging that there is no risk of mosquito genes affecting other species or getting into the food chain could help alleviate this concern.”
Take-Home Message
In sum, WNV is still highly relevant to the equine community and important for owners to keep in mind when managing their horses, regardless of season.
“Our climate is different than it used to be, and WNV is anticipated to hit new places as the birds are moving the virus to places where the disease hasn’t been centered before,” says Pelzel-McCluskey. “Plus, the change in climate is supporting the vectors, the mosquitoes, for extended periods of time. The good news is our vaccines work great for preventing WNV, and the vaccine is very affordable. I highly recommend owners not skip this recommended core vaccine.”
Related Reading
- A Refresher on Protecting Horses Against Arboviruses
- Rising EEE and WNV Cases Pose Equine and Public Health Risks
- Researchers Identify Potential New Transmission Method for West Nile Virus
Stay in the know! Sign up for EquiManagement’s FREE weekly newsletters to get the latest equine research, disease alerts, and vet practice updates delivered straight to your inbox.




