This article originally appeared in the Winter 2024 issue of EquiManagement. Sign up here for a FREE subscription to EquiManagement’s quarterly digital or print magazine and any special issues.

As a veterinary student in the 1980s, Stephanie Valberg, DVM, PhD, DACVIM (LAIM), DACVSMR, realized the profession knew little about how horses’ bodies function during exercise and what parameters should be used to measure fitness. She dreamed of pursuing a career in large animal medicine; however, her own autoimmune disorder put an abrupt end to that path. That didn’t diminish Valberg’s passion to work with horses and learn about exercise physiology, training, and muscle. Fueled by the promise of a treadmill and scholarship, Valberg jumped at the opportunity to begin her career in academia in Sweden.
“I had amazing mentors with a device that few others had—a muscle biopsy tool as well as a high-speed treadmill,” she recalls. “We were able to look at what happened in muscle when horses exercise and adapt to training.”
Valberg’s research in Sweden generated seven publications, and after earning her PhD, she returned stateside to continue her work in muscle research at the University of California, Davis. As we know, this was the start of a long and distinguished career directly impacting the lives of thousands of students, horses, and owners and culminating in a lifetime achievement award from the American College of Veterinary Internal Medicine in 2024.
Not One Muscle Disease
“There were two main draws to California for me,” says Valberg. “First, Dr. Gary Carlson had a strong interest in muscle disease, and Dr. George Cardinet III had a muscle biopsy laboratory for small animals and was working on staining these biopsies using techniques he learned from human medicine. I learned how to read muscle biopsies and started investigating horses that had symptoms of muscle disease.”
Her goal was to understand more specifically what happened with “muscle disease.”
“At that time, we were told there was one basic muscle disease called tying-up that occurred because of lactic acid buildup in the muscle,” says Valberg.
What didn’t make sense to her was why muscles would want to “kill themselves off” with lactic acid. Her research quickly led to the discovery that lactic acid accumulation was not a true explanation and there was more to the picture.
“Dr. Cardinet had a muscle biopsy from a horse called ‘Spook’ that contained an abnormal polysaccharide that he examined before I came to the University of California, Davis,” Valberg explains. “We set about using the muscle biopsy tool to find more horses and characterize the disease.”
In 1992, Valberg and colleagues published the first study reporting the existence of polysaccharide storage myopathy as a cause of chronic exertional rhabdomyolysis (tying-up) in horses.
“It is likely that a variety of etiological factors may contribute to the development of the clinical syndrome of exertional rhabdomyolysis,” they wrote, opening the flood gates of progress.
In that pinnacle study, Valberg collected muscle biopsies from nine horses with clinical evidence of recurrent exertional rhabdomyolysis (RER), including compatible history, clinical signs, and elevated serum creatine kinase (CK), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) activities—hallmarks of the condition. The research team examined the muscle biopsies, describing their histological, biochemical, and ultrastructural characteristics. They found high muscle glycogen concentrations, with up to 5% of type 2 muscle fibers containing inclusions that stained positively with periodic acid Schiff (PAS) stain for polysaccharide. Those inclusions contained beta glycogen particles interspersed among filamentous material. Valberg et al. compared these findings to a polysaccharide storage myopathy (PSSM) of unknown origin in human literature and found “strong evidence that some alteration in the metabolism of glycogen plays an important role in the pathogenesis of at least one form of equine exertional rhabdomyolysis.” They suggested this could be a result of an undefined metabolic disorder of skeletal muscle.
Breed-Specific Muscle Condition
In that 1992 study, Valberg et al. acknowledged that PSSM was likely the age-old form of tying-up in draft horses called “Monday morning disease,” yet the population of horses in the PSSM study were Quarter Horses, Quarter Horse crosses, American Paints, and Appaloosas.
“A study by Dr. Beth Valentine subsequently confirmed that PSSM was also present in Belgian and Percheron draft breeds,” Valberg adds.
Further, in 1999 her team reported that exertional rhabdomyolysis had different etiologies between Quarter Horses and Thoroughbreds. Scientists evaluated 18 Quarter Horses and 18 Thoroughbreds examined for RER at the Veterinary Teaching Hospitals at the Universities of California, Davis, and Minnesota, performing various tests, including muscle biopsy for muscle glycogen concentrations. They found that none of the Thoroughbreds but 15 of the 18 Quarter Horses with exertional rhabdomyolysis had abnormal polysaccharide accumulation consistent with PSSM. This confirmed that a glycogen storage disorder frequently causes exertional rhabdomyolysis in Quarter Horses, but a glycogen storage disease was not the cause of RER in Thoroughbreds. Rather, RER appeared to be due to abnormal intramuscular calcium regulation. Indeed, exertional rhabdomyolysis had multiple causes.
For this discovery, Valberg received the EquiSci International award.
“After this, we started collecting muscle biopsies from every horse with tying-up that we could,” she says. “We quickly learned there were different things going on in different breeds, and I really began focusing on PSSM in Quarter Horses and RER in Thoroughbreds and trying to understand what exactly was causing each of these diseases.”
Focus on PSSM
In 1993, Valberg moved into a faculty position at the University of Minnesota, partnering with Dr. Jim Mickelson, a driving force in the biochemical and genetic aspects of her research.
“When we looked at pedigree, we noticed that many of the horses with PSSM were related,” she explains. “Mickelson and I collaborated with scientists from around the world, building out our tools for genetic research. And after years of work, we found the genetic mutation for what we now refer to as type I PSSM (PSSM1).”
Those hard-won results were published in a 2008 study (McCue et al.), finally solving the mystery of what causes excess glycogen accumulation in the fast-twitch muscle fibers of horses with PSSM. McCue, Valberg, and Mickelson conducted a genome-wide association analysis on 48 related PSSM Quarter Horses and 48 unrelated control Quarter Horses using one of the first genome maps for horses. They isolated the probable cause to a small area on equine chromosome 10. At that time, the whole equine genome had not been sequenced, so they looked at a comparative region in the human genome containing the glycogen synthase-1 gene (GYS1) known to play a direct role in carbohydrate metabolism, encoding the skeletal muscle isoform glycogen synthase. This enzyme catalyzes the addition of glucosyl residue from UDP-glucose onto a glycogen polymer using an alpha-1,4 glycosidic linkage and is the rate-limiting step in glycogen synthase.
The team sequenced GYS1 from a single PSSM Quarter Horse and a control horse and identified a single G to A base substitution (single polymorphism) that changed arginine to histidine at codon 309. This Arg309His mutation was subsequently identified in 99 Quarter Horses with PSSM, with 72 horses heterozygous for the mutation and five homozygous. The remaining 22 were homozygous for the normal arginine allele.
In sum, these results pointed to a dominant gain-of-function mutation in GYS1 that results in enhanced activity of the mutant enzyme.
“Sixteen years later, we’re now able to pull some hairs and tell owners, ‘Your horse has type I PSSM,’” Valberg says.
Dietary Management of PSSM1: Additional research using a herd of horses with PSSM1 at the University of Minnesota ultimately found the best way to manage these horses is by feeding a low-starch and -sugar diet and providing dietary fat as an additional energy source. Working with Dr. Joe Pagan at Kentucky Equine Research, they developed a concentrate called Re-LEVE that was one of the first low-starch, high-fat feeds on the market. Insulin, which is released with high-starch and -sugar meals, activates the glycogen synthase enzyme, so it is important to keep insulin levels low. Regular daily exercise in some form is also important to enhance horses’ ability to burn glycogen and fats as fuel.
IMM: Another Muscle Disease of Quarter Horses
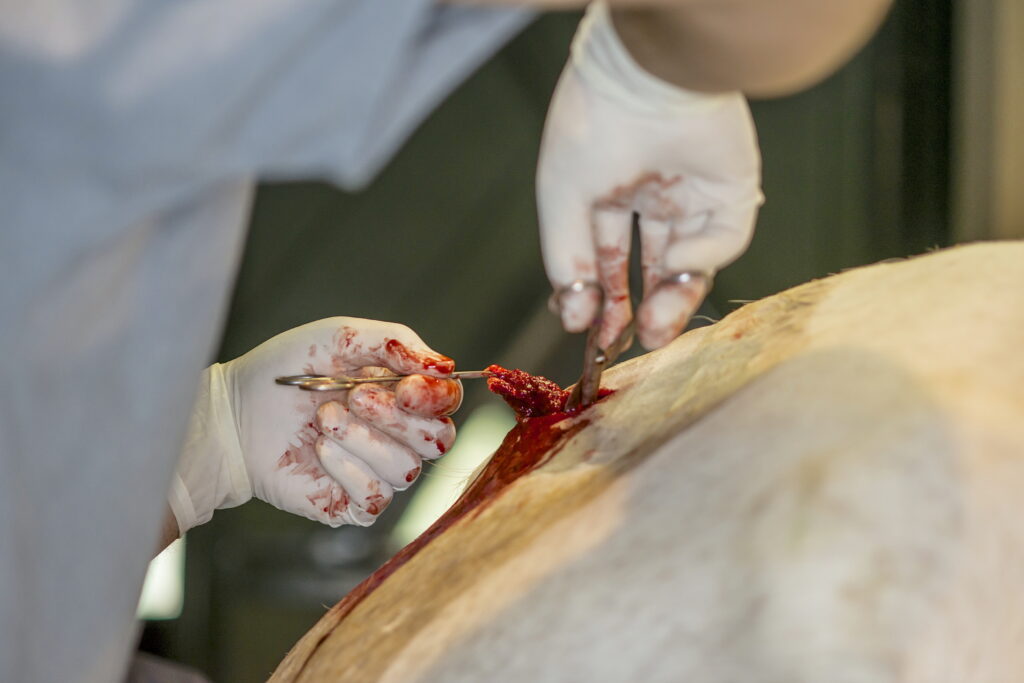
“Over the course of my career, I accumulated biopsies from over 10,000 horses,” Valberg says. “I had biopsies from many sad stories, including young Quarter Horses that developed severe muscle damage or muscle wasting and we could never figure out what was wrong with them.”
These Quarter Horses were losing all their muscling rapidly, over the course of only two to three days. Infectious disease, such as strangles, or a recent history of being vaccinated seemed to trigger it.
“The muscle biopsies showed an immune-mediated process because of the presence of specific lymphocyte cells infiltrating the myofibers,” says Valberg.
A closer look at this immune-mediated myositis (IMM) suggested a genetic predilection.
One of Valberg’s previous trainees, Carrie Finno, DVM, PhD, Dipl. ACVIM, collaborated and eventually found an underlying genetic cause of IMM in Quarter Horses, publishing those results in 2018. In that study, Finno’s team identified and assessed 36 IMM Quarter Horses and 54 unaffected Quarter Horses from the same environment.
Pedigree analysis showed all affected horses could be traced back to a common sire within eight generations, and either an autosomal dominant or recessive mode of inheritance was likely. The researchers performed genome-wide association and found nine single nucleotide polymorphisms on chromosome 11 to be significant. Further sequencing found that one of these genetic variants was present in all the IMM horses. It was located in MYH1, encoding the myosin heavy chain 2X. Protein modeling indicated this genetic mutation likely impacted the bonds and interactions between 14 amino acid residues in the MYH1 protein.
This is the first report of a MYH1 genetic mutation associated with muscle disease in any species. This mutation makes horses susceptible to IMM, but they do not all show disease signs. Instead, the researchers stated, “Within a particular environment, the MYH1 mutation results in invasion and destruction of type 2X myofibers by lymphocytes and rapid onset gross muscle atrophy.” It is a codominant mutation, meaning homozygous horses are highly likely to have pronounced clinical signs and about 20% of heterozygous horses develop less severe signs of muscle disease.
With the genetic mutation for IMM in hand, Valberg went back to her database of muscle biopsies to see if the MYH1 mutation impacted other muscles diseases. She discovered horses with this mutation also developed severe—and often fatal—rhabdomyolysis not induced by exercise. Additionally, some Quarter Horses with the MYH1 mutation developed atrophy and then calcification of a number of organs—also often fatal.
Valberg used the term myosin heavy chain myopathy (MYHM) to describe the three forms of disease associated with the MYH1 mutation, IMM, nonexertional rhabdomyolysis, and systemic calcification.
More Than One PSSM
As Valberg continued to examine muscle biopsies from Quarter Horses, it became clear that not all horses with abnormal-looking glycogen in muscle biopsies had PSSM1 due to the GYS1 mutation. In fact, 22 of the Quarter Horses diagnosed with PSSM in the original study that identified the GYS1 mutation did not have the GYS1 mutation. Valberg and colleagues referred to this as type 2 PSSM (PSSM2).
“While at first glance this was thought to be one disease, a breed-specific approach to the research revealed that there were several diseases that all had slightly abnormal-appearing glycogen in muscle biopsies,” Valberg relays.
After accepting the role of Mary Ann McPhail Dressage Chair at Michigan State University, Valberg directed her energies on discovering more about PSSM2. This involved taking a breed-specific approach and hypothesizing that PSSM2 likely encompassed several diseases.
In Quarter Horses, Valberg found that PSSM2 causes exertional rhabdomyolysis with high serum CK and AST activities similar to PSSM1. Glycogen concentrations are high in these Quarter Horses like they are in PSSM1.
“The term PSSM2-ER best describes these horses,” says Valberg, adding they’ve yet to identify the specific gene causing PSSM2-ER.
Dietary Management of PSSM2: This condition can be managed in a similar fashion to PSSM1.
Tying-Up in Arabians
“Around 2015 we shifted from PSSM1 and 2 in Quarter Horses and started looking at Arabian horses that started tying-up at the end of 100-mile rides,” says Valberg. “They seemed fine immediately after the rides but then developed myoglobinuria and rhabdomyolysis.”
Working with Dr. Erika McKenzie, Valberg collected and examined muscle biopsies from Arabians—either those prospectively diagnosed with tying-up or banked samples with a history of tying-up from the University of Minnesota’s Neuromuscular Diagnostic Laboratory. They also collected muscle samples from control horses residing on the same farm as the prospectively enrolled ER Arabians.
They evaluated these muscle biopsies for their light microscopic and electron microscopic appearance and biochemical measurements of the amount of glycogen.
The key findings on histopathology were centrally located nuclei in the mature myofibers, instead of the normal location of nuclei under the cell membrane. Some of the affected horses were diagnosed with PSSM2 based on a few fibers with increased staining for glycogen. Biochemical measurement of the amount of glycogen, however, did not find an increase in the total concentration of glycogen, indicating this was not a glycogen storage disease. Rather, ultrastructural studies found disrupted alignment of contractile proteins (myofibrils) and an accumulation of abnormal protein in some fibers in the prospectively enrolled Arabians with tying-up. The abnormal protein turned out to be clumping (aggregates) of a structural protein called desmin, which was found in both groups of Arabians with tying-up compared to controls.
“So, while many of the Arabians with banked muscle samples were previously diagnosed with PSSM2, this turned out to not be a glycogen storage disease as glycogen concentrations were normal in this study,” says Valberg.
Instead, she noted the false appearance of increased cytoplasmic glycogen in some muscle fibers, which might be because glycogen pools in areas where the contractile proteins are disorganized. Due to the disorganized areas of myofibrils and the accumulation of the structural protein desmin, Valberg called this condition myofibrillar myopathy (MFM-ER).
“MFM-ER is one form of exertional rhabdomyolysis in endurance Arabians,” she explains. “Other forms of exertional rhabdomyolysis in this breed include sporadic exertional rhabdomyolysis due to nutritional imbalances or a lack of conditioning and RER, which usually occurs at the beginning of endurance rides when horses are fit and stressing to go faster.”
Subsequent gene expression and proteomic analysis of muscle from MFM-ER Arabians before and after exercise implicated oxidative stress, which can cause desmin to aggregate. Further, the lack of a specific form of antioxidants—cysteine-based antioxidants—contributes to the development of exertional rhabdomyolysis in these horses.
Dietary Management of PSSM1: A specific nutritional supplement (described below) was developed for MFM in collaboration with Dr. Joe Pagan at Kentucky Equine Research. In addition, Arabians with MFM-ER must have regular daily exercise to avoid tying-up episodes.
Passion Project Prior to Retirement: MFM in Warmbloods

As retirement drew near, Valberg recognized a void in her research: Warmbloods.
“I was getting more and more biopsies from warmbloods that were originally doing great in training then started to not want to move forward, were becoming resistant to exercise, had no desire to go, and could not maintain a canter,” says Valberg.
Those warmbloods had no evidence of classic tying-up and no elevation in CK. Full diagnostic work-ups revealed no major lameness, no kissing spines, no ulcers … nothing.
“These horses were often diagnosed with PSSM2 based on mild abnormalities in the appearance of glycogen under the microscope,” she explains. “However, when we measured the amount of glycogen, like the Arabians, glycogen concentrations were normal.”
In a 2017 study, Valberg et al. found that muscle biopsies of warmbloods previously diagnosed with PSSM2 showed desmin aggregation in some fibers accompanied by regions with Z disc and myofibrillar disruption similar to the findings in Arabian horses with MFM. So, while the clinical signs were very different between warmbloods and Arabians, the microscopic findings were very similar.
A follow-up study published in 2021 (Williams et al.) used novel techniques at that time, proteomics and transcriptomics, as well as immunofluorescence and electron microscopy, to further characterize MFM in warmbloods. MFM was then described as a “late-onset disease of unknown origin characterized by poor performance, atrophy, myofibrillar disarray, and desmin aggregation in skeletal muscle.”
Muscle biopsies were obtained from warmbloods with histories of poor performance and exercise intolerance not attributable to lameness and control horses with no history of performance issues residing within a 15-mile radius of the affected warmbloods.
“There were striking differences between control and MFM warmbloods reflected by the number of proteins and genes that had different degrees of expression,” says Valberg. “The differentially expressed genes and proteins were not identical to those found in Arabians.”
One of the top differentially expressed proteins was CSRP3, which is integrally involved in Z-disc signaling, gene transcription, and sarcomere integrity. Immunohistochemistry localized CRSP3 to type 2A muscle fibers, fast-twitch oxidative fibers. CSRP3 has been referred to as a master regulator of muscle, and it plays a major role in sensing and producing training adaptations to exercise.
On the DNA side, the top differentially expressed gene was CHAC1 encoding a protein that degrades glutathione, a ubiquitous cysteine-based antioxidant associated with oxidative stress and apoptosis.
Valberg did not find significant variants in genes encoding amino acids associated with MFM, so a simple genetic basis for the disease was not identified.
“The commonality between Arabians and warmbloods with MFM was the histologic appearance of desmin aggregates and the role of oxidative stress and a lack of cysteine-based antioxidants,” she adds. “There are also significant differences that appear to indicate that they have different forms of MFM.”
In endurance Arabians, Valberg believes desmin aggregation and rhabdomyolysis likely result from a nutritional deficiency of cysteine-based antioxidants. During endurance races, horses metabolize a lot of fat, which creates oxidative stress and, if horses lack cysteine-based antioxidants, results in rhabdomyolysis with oxidation of the highly sensitive protein desmin by the end of the ride. In warmbloods, molecular signs of an abnormal adaptation of skeletal muscle to training were predominant, and there might be continual and unnecessary breakdown of the essential cysteine-based antioxidant glutathione. For warmbloods with MFM, Valberg recommends a training approach that gives muscle time to recover and adapt to exercise—two days off and then three days of training as opposed to daily training.
Dietary recommendations: Valberg worked with Dr. Pagan at Kentucky Equine Research to find a way to increase cysteine-based antioxidants in skeletal muscle after exercise. They developed the MFM pellet that contains amino acids as well as N-acetylcysteine, one of the few ways to increase glutathione concentrations in muscle.
“We have seen an incredible response to these diet and training recommendations in MFM horses,” Valberg states. “To me that is so rewarding.”
A Career in Review
When asked what Valberg was most proud of during her incredibly successful career, she says, “Being able to collaborate with a lot of amazing scientists. Together we were able to go from the lab bench right to assisting veterinarians diagnose the disease and helping horse owners manage the disease and make the horse rideable … from basic science to changing veterinary practice and changing how owners enjoy their horses.”
While Valberg has been part of many exciting discoveries, she says one of the most incredible feelings in her career came the day she was 100% sure she found the gene causing PSSM1 and later the gene causing MYHM.
“There are millions of genetic variants in a horse’s genome, most of which do not cause a disease,” she explains. “For decades you search and find it could be this one but it’s not, and it’s not this one and not that one, but when you finally find the one and show that it is actually causing the disease, it’s very exciting. It is so important to make sure that a variant you discover it is actually the genetic variant that causes the disease, and that takes time and a lot of research.”
Passing the torch to her successors, Valberg says, “Many equine muscle diseases have a complex interaction between numerous genes, diet, and training. As research tools continue to evolve, I look forward to seeing these complexities solved and how they will lead to better diagnostic tests and treatments for muscle disorders.”
Related Reading
- Diagnosing Performance-Limiting Muscle Diseases in Horses
- Type 2 PSSM in Quarter-Horse-Related Breeds
- Managing Horses With Low Serum Vitamin E
Stay in the know! Sign up for EquiManagement’s FREE weekly newsletters to get the latest equine research, disease alerts, and vet practice updates delivered straight to your inbox.