In veterinary medicine wounds are common, ranging from simple scrapes, surgical incisions, and dermatologic issues to serious life and limb threatening traumas. The body begins work immediately upon insult to the skin to repair damaged tissue and restore the functional barrier properties of the skin.
Equine wound management presents many problems for the practicing veterinarian.1 Microbial contamination and biofilm growth are common and can prevent healing. Tensional forces on the wound can lead to wound expansion. Wound contraction is often slow, increasing the risk of developing proud flesh which slows or stalls healing and necessitates debridement. The challenges are exacerbated in the case of distal limb wounds which contract and re-epithelialize more slowly than truncal wounds. Slow healing and chronic inflammation can lead to weak and poorly perfused tissue, proud flesh, and potentially scarring.
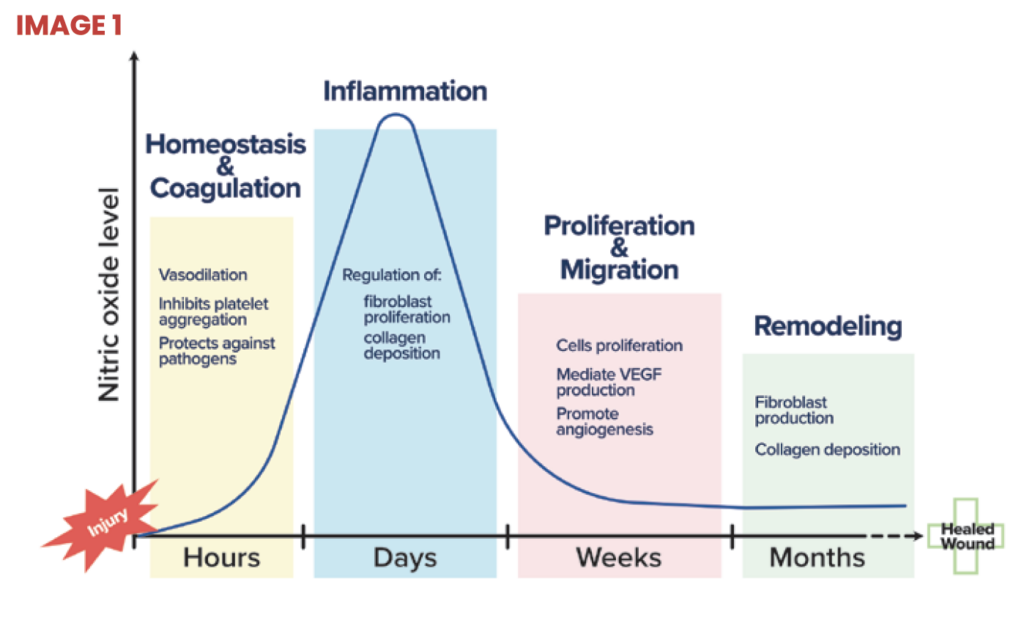
An ideal wound healing product would promote rapid healing and mitigate these issues, and should be able to be utilized without the need for specialized regimens, training or equipment, or high price tags. Therapeutic application of nitric oxide is one such candidate that has garnered significant attention due to its diverse and critical functions throughout eachpost-hemeostasis phase of the wound healing cascade (image 1).
Nitric oxide: A key regulator of the natural healing process
Nitric oxide is an endogenous gas that serves as a biologic messenger in many physiologic processes,2 most notably, wound healing.3 Nitric oxide is produced by enzymes acting upon the amino acid L-arginine. Nitric oxide as a topical treatment has shown tremendous promise across species in everything from human and equine to companion animal and exotic applications.4
Functions across all phases of wound healing:
Inflammatory phase – Nitric oxide acts as a signaling molecule that promotes the growth and activity of immune cells and the biochemical reactions which are used to defend against bacteria, fungi, viruses, and parasites. Additionally, nitric oxide induces broad spectrum damage to pathogens caused by nitrosative and oxidative reactions. Nitric oxide also controls immune cell signaling and the biochemical reactions which are used to defend against bacteria, fungi, viruses, and parasites. Further, nitric oxide controls macrophage polarization helping wounds transition from the inflammatory phase.
Proliferative phase – Nitric oxide drives increased blood flow, angiogenesis, proliferation, and epithelialization resulting in rapid wound closure, reduced proud flesh, and stronger tissue. Nitric oxide upregulates expression of endogenous collagenase, which autolytically debrides the wound, to further promote the healing process. Nitric oxide has been shown to stimulate the proliferation of endothelial cells, protect endothelial cells from apoptosis, and mediate vascular endothelial growth factor (VEGF) production. These effects of nitric oxide on endothelial cells guide angiogenesis, the formation of new capillaries. The resulting increased blood flow boosts the transport of proteins into the wound bed facilitating wound healing.
Remodeling phase – Low levels of nitric oxide increase keratinocyte proliferation. Nitric oxide coordinates increased collagen synthesis and deposition in the final phases of wound healing. Treatment with nitric oxide donors has been shown to increase collagen formation from fibroblasts.
Delivering nitric oxide
Multiple nitric oxide delivery systems for wound care have been developed. Despite the success of nitric oxide delivery systems in treating laboratory models of wounds and infection, practical use in the field has been hampered by high complexity and costs. A new electrochemical system has been introduced that overcomes the complexity and cost challenges. The pad and gel forms are activated by the addition of water. Once activated, a biologically relevant level of nitric oxide is produced over multiple days.
The first practical application of this approach has just been released by Noxsano in wound dressings that are optimized to deliver nitric oxide at a level that drives increased blood flow, angiogenesis, proliferation, and epithelialization resulting in improved wound healing. Independent clinical studies of Restore show 40% faster wound healing. Restore delivers faster granulation, reduction in total wound area, and increased percent contraction.5
Impact on equine wounds
In addition to driving healing in equine wounds, nitric oxide promotes processes that would be expected to mitigate the formation of proud flesh. The precise causes of proud flesh are not fully understood but recent literature6,7,8 points to several factors such as low perfusion (hypoxia) due to poor blood flow, lack of nutrition at the wound site related to the poor blood flow, and infection/biofilm formation. These factors are believed to result in the wound becoming stalled in the inflammatory phase. Nitric oxide delivered therapeutically will promote blood flow to the wound through dilation and angiogenesis, eliminate biofilm/infection, and polarize macrophages from pro- to anti-inflammatory to transition out of the inflammatory phase. Doing this can greatly reduce the occurrence of overgranulation and promote normal healing.
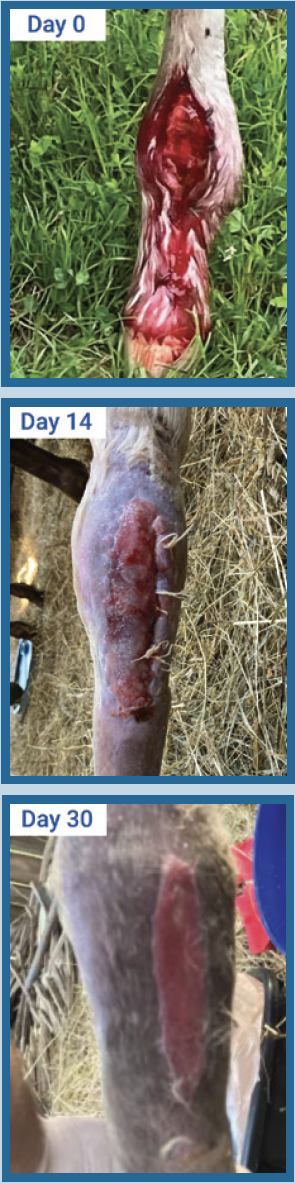
Wire cut fetlock case study
A wire cut fetlock was presented to the veterinarian who sutured the injury shut and overwrapped the wound. After 14 days, the wound dehisced and the veterinarian noted small amounts of proud flesh. Post light debridement the veterinarian decided to place a nitric oxide generating bandage on the wound. From that point he changed the bandage 2x/week for 2 weeks resulting in outstanding closure and a horse that was able to return quickly to work on the farm.
References
1. Ed. Theoret, C, Schumacher, J. 2016, Equine Wound Management (3), John Wiley & Sons, Ltd, doi: 10.1002/9781118999219
2. Howe LM, Boothe HW Jr. Nitric oxide: a review for veterinary surgeons. Vet Surg. 2001 Jan-Feb;30(1):44-57. doi: 10.1053/ jvet.2001.20341. PMID: 11172460.
3. Witte MB, Barbul A. Role of nitric oxide in wound repair. Am J Surg. 2002 Apr;183(4):406-12. doi: 10.1016/s0002-9610(02)00815-2. PMID: 11975928.
4. Sumner, SM, Wallace, ML, Mulder, AT, Delmotte, SB, Duran, SH, Lindell, H, Annaji, M, Dimick, T, Poudel, I, Babu, JR. Development and evaluation of a novel topically applied sildenafil citrate hydrogel and its influence on wound healing in dogs Am J Vet Res. 2022 Aug; doi: 10.2460/ajvr.21.12.0209
5. Data per UGA College of Veterinary Medicine Professor Mandy Wallace and surgical resident Dr. Jenniffer Rodriguez-Diaz as presented at the Society for Veterinary Soft Tissue Surgery (June 2023 Jacksonville, FL) and Southern Veterinary Conference (July 2023 Birmingham, AL) and article in progress.
6. Alhajj M, Goyal A. Physiology, Granulation Tissue. [Updated 2022 Oct 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/ NBK554402/
7. Deschene, K, et al., Hypoxia regulates expression of extracellular matrix associated proteins in equine dermal fibroblasts via HIF1, Journal of Dermatological Science, 2012, 65 Issue 1, pg 12 – 18 doi: 10.1016/j. jdermsci.2011.09.006
8. Dart AJ, et al. Selected factors that negatively impact healing. Equine Wound Management Oct 18th, 2016. : https://doi. org/10.1002/9781118999219.ch3